Looking back at the amazing EBM Congress in Vienna!
EffectiveBioMedicine Congress in Vienna 2025 We are incredibly proud to have been part of the Effective BioMedicine Congress2025 – not only as e...
The device as well as the production are certified according to the EC guidelines 93/42/EEC Annex II as well as EN ISO 13485.

papimi is a certified and approved medical device of class IIa.
The American patent was granted on September 17, 1996 (US 5,556,418). The first European patent (EP 0648.140.B1) was granted on January 31, 2001 and the second (EP 04.743.704.1) in August 2003.
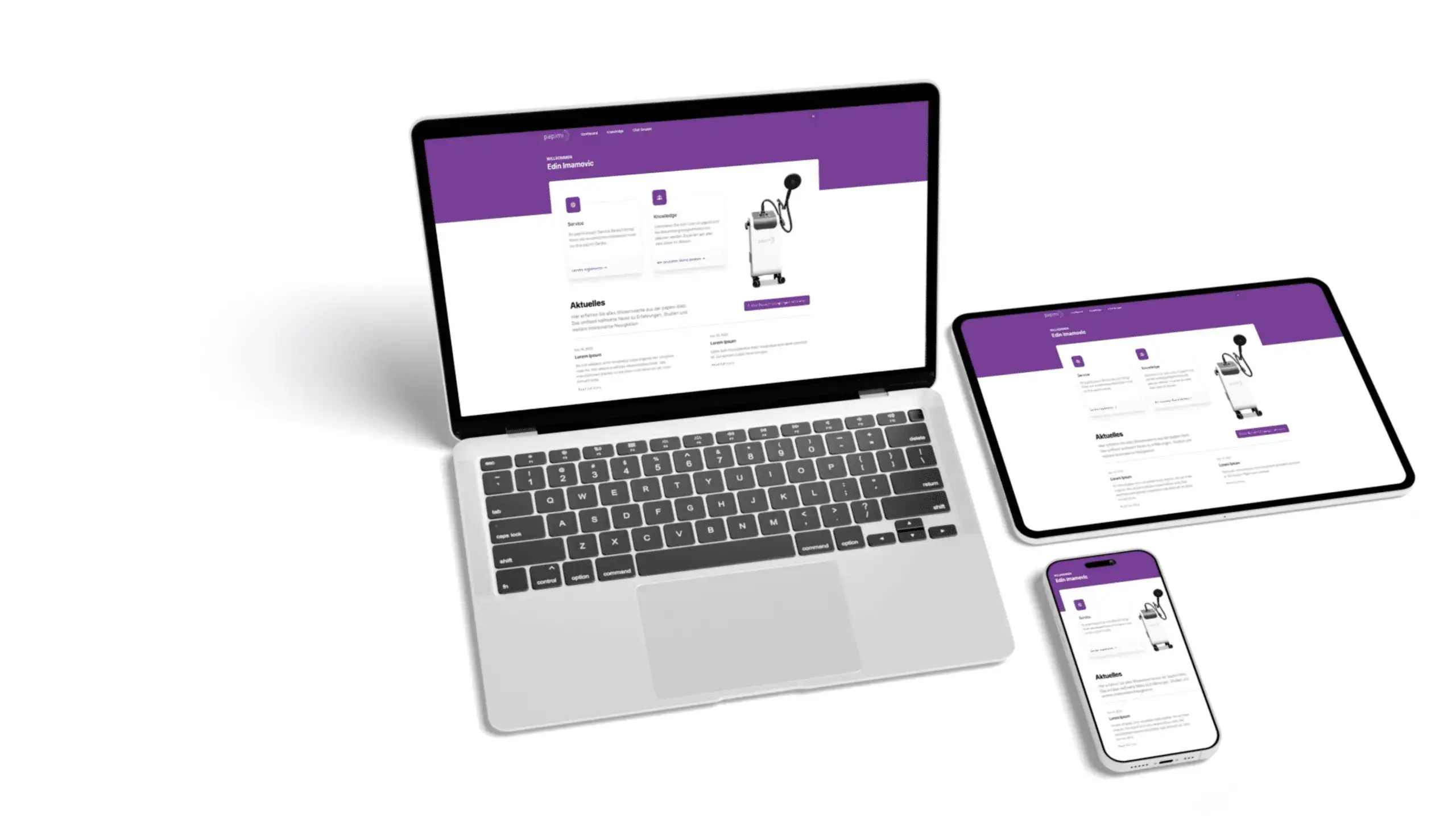
Our premium service ensures that your device always performs flawlessly. Our staff will also inform you about our many warranty options.
The device as well as the production are certified according to the EC guidelines 93/42/EEC Annex II as well as EN ISO 13485.

papimi is a certified and approved medical device of class IIa.
The American patent was granted on September 17, 1996 (US 5,556,418). The first European patent (EP 0648.140.B1) was granted on January 31, 2001 and the second (EP 04.743.704.1) in August 2003.
Our premium service ensures that your device always performs flawlessly. Our staff will also inform you about our many warranty options.
With the papimi DELTA, Ion-Induction-Therapy (IIT) has arrived in the 21st century. This Physical Cell Therapy sets new standards in medicine. Learn more about the Original by Prof. DDr. Pappas.

Spiral-applicator XL
Ring-applicator
Spiral-applicator M
Ion-Induction-Device with applicator-arm

With the papimi DELTA, Ion-Induction-Therapy (IIT) has arrived in the 21st century. This Physical Cell Therapy sets new standards in medicine. Learn more about the original by Prof. DDr. Pappas.













EffectiveBioMedicine Congress in Vienna 2025 We are incredibly proud to

Case Report: Refractory Seizures Controlled with papimi IIT in Patient

papimi IIT & Prostate Health: What Clinical and Practical Experience

New Study: papimi Reduces Pain and Balances Nervous System —
EffectiveBioMedicine Congress in Vienna 2025 We are incredibly proud to have been part of the Effective BioMedicine Congress2025 – not only as e...
Case Report: Refractory Seizures Controlled with papimi IIT in Patient with Benign Brain Tumor A 39-year-old woman with a non-operable lipoma in the c...
papimi IIT & Prostate Health: What Clinical and Practical Experience Show Can a non-invasive, painless method offer support for men with benign pr...
New Study: papimi Reduces Pain and Balances Nervous System — After Just One Session 🧬 New Study: papimi Reduces Pain and Balances Nervous System ...
The papimi technicians team welcomes three new experts from Croatia and the UK! Fully trained, they are now providing top-level support worldwide....
New specialist article available: How is Ion-Induction-Therapy (IIT) with papimi transforming modern dentistry? Find out in "OM & Nutrition 2024." Rea...
NieCo 2024 exceeded expectations with its stellar lineup of speakers, engaging seminars, hands-on workshops, and a fantastic venue, making it an aweso...
2023 completed We will be back on the 8th of January 2024 (For technical support during this time, please call our headquarters in Athens directly: +3...
Medicine needs to be effective, and so are the connections we've woven. 🌐 Let's continue to be a catalyst for collaboration, innovation, and effect...
Last weekend, our "Integration of #BiologicalDentistry, #Osteopathy, and #Postural #Medicine" seminar took place at the MAHA Integrative Medicine ...